Understanding the quality of the air we breathe is essential for a sustainable future. With our innovative air quality monitoring kit, connected to a computer, we can collect and analyze data in real time. This hands-on approach allows students to explore the impact of human activity on the environment and develop solutions for healthier cities.




Here are the real-time results from our sensors:
Air pollutants are numerous. They can be classified into several categories:
- chemical
- physical
- biological
Chemical polluants
Physical polluants

Biological Polluants

Meteorology
Air quality is closely linked to meteorological phenomena. Meteorology influences the transformation, dispersion, accumulation and deposition of pollution.

Temperatures affect the volatility of compounds, dispersion (by convection) and emissions of pollutants (increased sources in cold periods).
Solar radiation alone can transform certain compounds into others. This is the case with ozone, which is formed from nitrogen oxides and COV under the action of solar radiation

Under normal conditions, the temperature decreases with altitude. The warmer air on the ground naturally carries with it the pollutants emitted on the ground. The concentrations of these pollutants decrease and disperse at higher altitudes.
Under certain conditions, this temperature gradient is broken and colder air is present on the ground. Natural convection no longer takes place and the pollutants accumulate.

Wind moves air masses and the pollutants they contain. It can transport pollution or, on the contrary, disperse pollutants.

Precipitation ‘leaches’ pollutants from the atmosphere by washing them down to the ground, reducing their concentration in the air. On the other hand, it can transfer pollutants into the soil or water.
Qualité de l’air

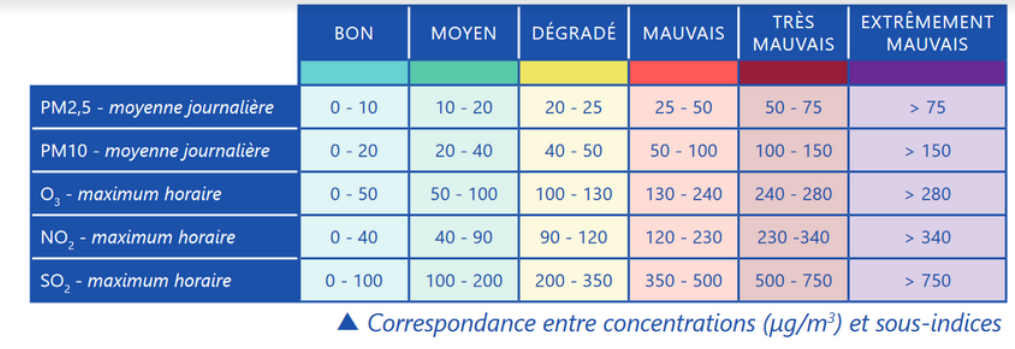
The index is constructed by integrating 5 different pollutants:
– fine particles (PM10)
– fine particles (PM2.5),
– nitrogen dioxide (NO2),
– ozone (O3),
– sulphur dioxide (SO2).
All these polluants have impacts on our health and our environment.
VOCs are produced by industrial processes:
- chemicals, metal degreasing, paint, printing, glues and adhesives, rubber, cleaning products, perfumes and cosmetics, etc.
- oil refining, production of alcoholic beverages, bread, etc.
- industrial combustion, etc.
VOCs are also emitted naturally: forests, Mediterranean vegetation, cultivated areas, fermentations, etc.